Models of Host-Pathogen Dynamics
Epidemiological models are sets of differential equations that quantitatively describe how pathogens spread among infectious and susceptible host organisms. Mathematical analysis of these models has led to deep and general insights about disease transmission, including mechanisms of epidemic spread, and critical thresholds for disease control (e.g., vaccination and culling). Disease models are also routinely used to forecast pathogen spread in plant, wildlife, and human systems under future and novel scenarios.
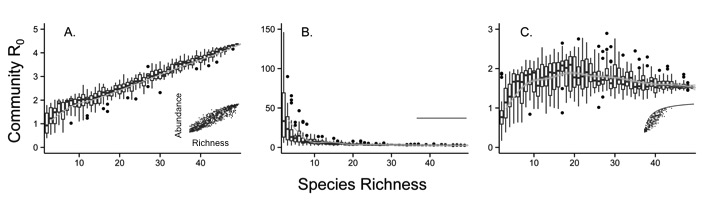
By developing generalized models, we can better understand phenomena that apply to many systems. For instance, much of our lab’s prior research has focused on developing theory to understand how biodiversity affects the transmission of multi-host pathogens (e.g., those that cause zoonotic disease in humans). In doing so, we’ve developed more quantitative expectations for when more speciose communities should buffer against disease spread, and when they should enhance disease spread.
In contrast, by constructing models that are specific to certain disease systems, we can simultaneously generate biological insights with the benefit of making detailed disease forecasts. Our goal is to integrate data from multiple sources to inform our models, so that we can make meaningful, quantitative predictions for wildlife and human health, and for pest control. Thus, our lab is also focused on developing statistical computing methods that allow us to flexibly infer the parameters of mathematical disease models from various data types:
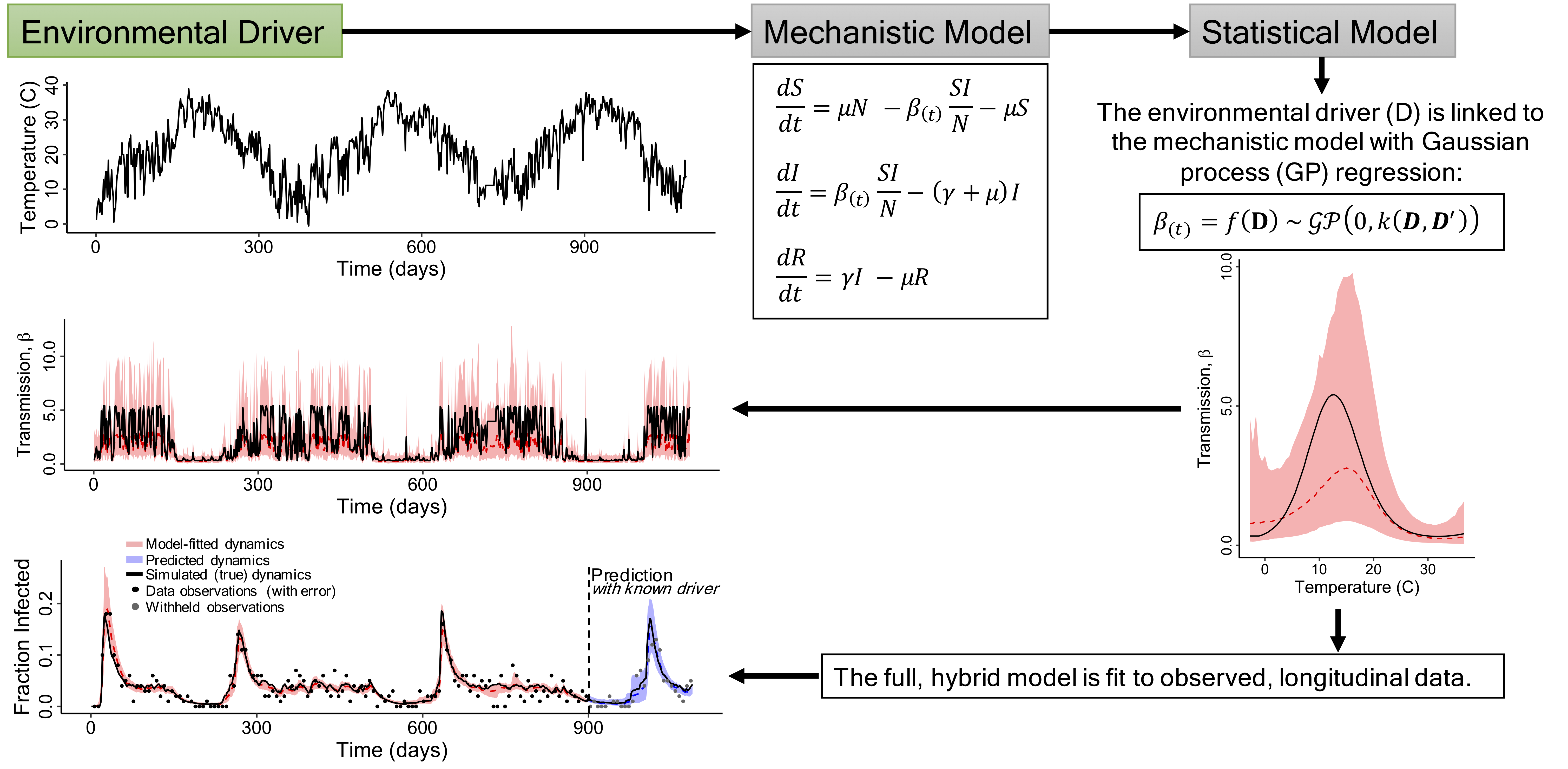
In this way, our data informs our models, and our parameterized models can make predictions. Some of our work involves modeling the spread of viruses used for biocontrol, and more information about those projects can be found here.
Amphibian Ranaviruses as a model system
In addition to insect viruses, we use amphibian viruses as a model system to better understand host-pathogen dynamics in a changing world. The genus Ranavirus is an ecologically and genetically diverse clade of double-stranded DNA (dsDNA) viruses. Ranaviruses are globally widespread, and regionally prevalent, which makes them a tractable system to explore fundamental questions in disease ecology, epidemiology, and conservation biology.
We are using epidemiological modeling to better understand the within- and among-host processes that affect Ranavirus transmission, with the goal of developing methods that can be broadly applied to other disease systems. For instance, we have fit models that describe the interactions between viral replication and immune-system reponses to infer the mechanisms that control the within-host population dynamics of Ranaviruses:
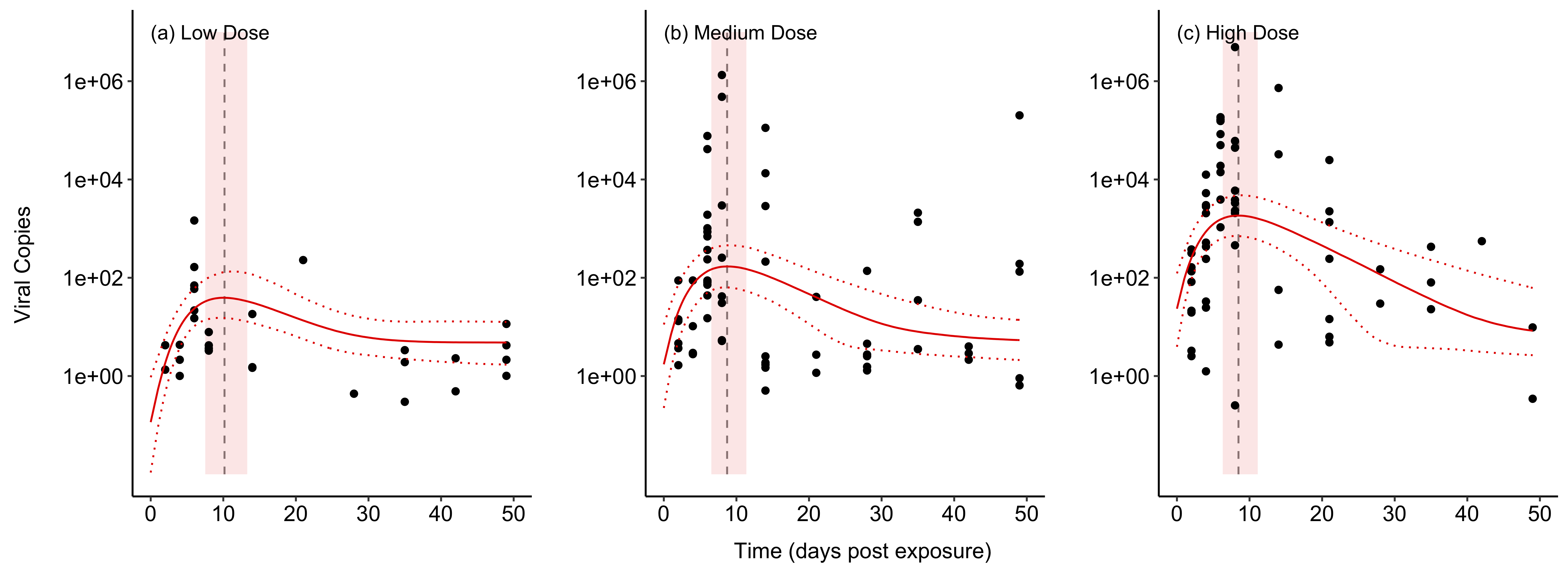
Our current work is focused on developing flexible models of viral transmission in amphibian populations, with the goal of integrating the effects of environmental temperature. In this way, we can use our models to forecast disease transmission under future and novel climate conditions.
